Information about COVID-19 (Coronavirus) is being released rapidly. We are posting updates here as we get them.
COVID-19 Resources:
EBAA COVID-19 Update Webinar (4/3/2020)
Center for Disease Control and Prevention
Center for Disease Control Travel Health Notice site
World Health Organization
EDQM Webinar: Tissue Donation from Deceased Donors (04/28/2020)
Regulatory updates
These recommendations have been updated as of March 14, 2022. Please contact Jennifer@RestoreSight.org with questions.
March 14, 2022
UPDATED GUIDANCE AND COVID-19 SCREENING RECOMMENDATIONS
Download Updated Guidance Here
The Policy & Position Review Subcommittee of the EBAA Medical Advisory Board continues to update its guidance and screening recommendations as the COVID-19 pandemic evolves based on the latest guidelines from the FDA and CDC as well as available scientific evidence. The most current set of guidelines, designed for recipient and recovery technician safety, specify criteria for: 1) Donor ineligibility and 2) Donor eligibility requiring Medical Director review. In this second category, donors who have signs* and symptoms† possibly consistent with COVID-19 but that are reasonably explained by a plausible alternative etiology may be eligible for transplantation. Donors who are not excluded by one of the criteria listed below should be considered eligible if all other eligibility criteria are met.
- Donors should be determined ineligible who in the 10 days prior to death:
- were diagnosed with acute COVID-19; OR
- tested positive for COVID-19 by direct viral testing methods (e.g., NAAT and/or antigen); OR
- had close contact‡ with a person diagnosed with or suspected to have COVID-19 AND developed signs and symptoms of COVID-19, regardless of a plausible alternative etiology or vaccination history
- Donors should be evaluated for eligibility by a Medical Director who:
- in the 10 days prior to death, without a known close contact with a person diagnosed with or suspected to have COVID-19, developed new signs and/or symptoms consistent with acute COVID-19 not explained by a plausible alternative etiology; OR
- in the 10 days prior to death, had a known close contact with a person diagnosed with or suspected to have COVID-19 AND was asymptomatic; OR
- in the 11 to 20 days prior to death had a positive or reactive test for SARS-CoV-2 AND had ongoing signs and/or symptoms of COVID-19, regardless of a plausible alternative etiology.
NOTES
The value of donor screening for SARS-CoV-2 is subject to ongoing assessment. This guidance provides the means to minimize COVID-19 transmission risk and will allow for the continued provision of safe corneal tissue to patients while minimizing the wastage of suitable donor corneal tissue. Eye bankers and corneal surgeons should continue to keep in mind the following with regard to the safety of corneal tissue and ocular tissue recovery:
- Individuals who have received non-replicating, inactivated, or RNA-based COVID-19 vaccines are not precluded from donating cells, tissues, or cellular or tissue-based products.3 If the vaccination status of a donor is known, it must be communicated to end-users on Tissue Report Forms or other supporting documents.
- Current EBAA Medical Standards require use of a double povidone iodine donor prep; povidone iodine has documented in vitro viricidal activity against coronaviruses.
- The EBAA acknowledges that other associations, hospital systems, eye banks, departments of health, or governments may require that all donors be tested for COVID-19. Eye banks must establish a protocol to ensure access to testing notification and results obtained by partner agencies to prevent discordant resulting and/or discovery of results after release of tissue for transplant use. Results of such testing must be communicated to end-users on Tissue Report Forms or other supporting documents.
- Cadaveric PCR or antigen testing for SARS-CoV-2 may be an additional tool to assist Medical Directors in determining donor eligibility. However, currently available tests for detecting the SARS-CoV-2 virus have not been validated for postmortem use.4
- Medical Director review for final determination of donor eligibility in certain cases allows for further assessment of the full clinical picture and/or case specific scenarios.
- There have been no reported cases of transmission of SARS-CoV-2, MERS-CoV, or any other coronavirus via transplantation of ocular tissue.4
REFERENCES
1 Mayo Clinic Staff. “COVID-19 (Coronavirus): Long-term Effects”. Mayo Clinic Health System. October 22, 2021. https://www.mayoclinic.org/diseases-conditions/coronavirus/in-depth/coronavirus-long-term-effects/art-20490351
2 United States, Department of Health and Human Services, Centers for Disease Control and Prevention. “Post-COVID Conditions.” Centers for Disease Control and Prevention (US CDC). September 16, 2021. https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html
3Updated Information for Human Cell, Tissue, or Cellular or Tissue-based Product (HCT/P) Establishments Regarding the Coronavirus Disease 2019 Pandemic”. US Food & Drug Administration, US Department of Health & Human Services, January 4, 2021, https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/updated-information-human-cell-tissue-or-cellular-or-tissue-based-product-hctp-establishments.
4Aldave AJ, DeMatteo J, Chamberlain WD, Philippy B, Farooq AV, Buckman N, Crosson A, Li J, Meinecke E, Kaufman AH. “COVID and the Cornea: From Controversies to Consensus: Report of the Eye Bank Association of America Medical Advisory Board Policy and Position Review Subcommittee.” Cornea. 2021;40:809-816.
October 27, 2021
Download updated guidance and COVID-19 Screening recommendations.
June 3, 2021
GUIDANCE RATIONALE
The EBAA Policy & Position Review Subcommittee of the Medical Advisory Board continues to update guidance and screening recommendations as the COVID-19 pandemic evolves. Progression in our understanding of the utility of donor screening for the SARS-CoV-2 virus, the risk of transmission via corneal transplantation and means to minimize this risk will allow for the continued provision of safe corneal tissue to patients while minimizing the wastage of suitable donor corneal tissue. As we return to pre-pandemic levels of elective corneal transplantation procedures across the US, eye bankers and corneal surgeons should keep in mind the following with regard to the safety of corneal tissue:
- Individuals who have received non-replicating, inactivated, or RNA-based COVID-19 vaccines are not precluded from donating cells, tissues, or cellular or tissue-based products. 1 If vaccination status of a donor is known, it must be communicated to end-users on Tissue Report Forms or other supporting documents.
- Current Medical Standards of the EBAA requires use of a double povidone iodine donor prep; povidone iodine has documented in vitro viricidal activity against coronaviruses.
- The EBAA acknowledges that other associations, hospital systems, eye banks, departments of health, or governments may require that all donors be tested for COVID-19. Eye banks must establish a protocol to ensure access to testing notification and results obtained by partner agencies. Results of such testing must be communicated to end-users on Tissue Report Forms or other supporting documents.
- Medical Director review for final determination of donor eligibility in certain cases allows for further assessment of the full clinical picture and/or case specific scenarios.
- There have been no reported cases of transmission of SARS-CoV-2, MERS-CoV, or any other coronavirus via transplantation of ocular tissue.2
REFERENCES
1Updated Information for Human Cell, Tissue, or Cellular or Tissue-based Product (HCT/P) Establishments Regarding the Coronavirus Disease 2019 Pandemic”. US Food & Drug Administration, US Department of Health & Human Services, January 4, 2021, https://www.fda.gov/vaccines-blood-biologics/safety-availabilitybiologics/updated-information-human-cell-tissue-or-cellular-or-tissue-based-product-hctp-establishments.
2 Aldave AJ, DeMatteo J, Chamberlain WD, Philippy B, Farooq AV, Buckman N, Crosson A, Li J, Meinecke E, Kaufman AH. COVID and the Cornea: From Controversies to Consensus: Report of the Eye Bank Association of America Medical Advisory Board Policy and Position Review Subcommittee. Cornea. 2021 Mar 29. Epub ahead of print.
January 4, 2021
FDA continues to work closely with CDC and other federal and international agencies to monitor the coronavirus disease 2019 (COVID-19) pandemic caused by the virus, SARS-CoV-2. Respiratory viruses, in general, are not known to be transmitted by implantation, transplantation, infusion, or transfer of human cells, tissues, or cellular or tissue-based products (HCT/Ps). To date, there have been no reported cases of transmission of COVID-19 via these products.
Routine screening measures are already in place for evaluating clinical evidence of infection in HCT/P donors.
Considerations
FDA does not recommend using laboratory tests to screen asymptomatic HCT/P donors.
FDA is aware that some HCT/P establishments in the U.S. are considering additional donor screening and testing measures in response to the COVID-19 pandemic.
The HCT/P establishment’s responsible person must determine and document the eligibility of a cell or tissue donor (21 CFR 1271.50). Based on information available at this time, establishments may wish to consider, whether, in the 28 days prior to HCT/P recovery, the donor
- cared for, lived with, or otherwise had close contact with individuals diagnosed with or suspected of having COVID-19 infection; or
- had been diagnosed with or suspected of having COVID-19 infection; or
- had a positive diagnostic test (e.g., nasopharyngeal swab) for SARS-CoV-2 but never developed symptoms.
COVID-19 vaccines hold the promise to alter the course of this pandemic and are complex biological products intended to be administered to millions of individuals to prevent COVID-19. Recently, vaccines for the prevention of COVID-19 have become available under emergency use authorization (EUA) issued by the FDA. Based on information available at this time, those who have received non-replicating, inactivated, or RNA-based COVID-19 vaccines are not precluded from donating HCT/Ps.
FDA will continue to monitor the situation and will issue updates as information becomes available.
Additional Resources:
- CDC: Coronavirus Disease 2019 (COVID-19) web page
- FDA: Coronavirus Disease 2019 (COVID-19) web page
- FDA: Emergency Use Authorization for Vaccines to Prevent COVID-19: Guidance for Industry web page
October 20, 2020
GUIDANCE RATIONALE
The EBAA Policy & Position Review Subcommittee of the Medical Advisory Board continues to update guidance and screening recommendations as the COVID-19 pandemic continues to evolve rapidly. Developments in our understanding of this novel SARS-CoV-2 virus and in our ability to screen donors should allow for the continued provision of safe corneal tissue to patients during this time. As we again proceed with elective corneal transplantation procedures across the US, the safety of corneal tissue may be supported by the following:
- There have been no reported cases of transmission of SARS-CoV-2, MERS-CoV, or any other coronavirus via transplantation of ocular tissue. A recent study reported that no SARS-CoV-2-RNA was detected in the cornea, conjunctiva, or aqueous humor of five COVID-19 positive postmortem 1
- Current Medical Standards of the EBAA requires use of a double povidone iodine donor prep; povidone iodine has documented in vitro viricidal activity against
- Increased testing of patients in the hospital and outpatient settings for COVID-19, and greater understanding of COVID-19 symptoms will enhance donor screening and the safety of donor
- Medical Director review for final determination of donor eligibility in certain cases allows for further assessment of the full clinical picture and/or case specific
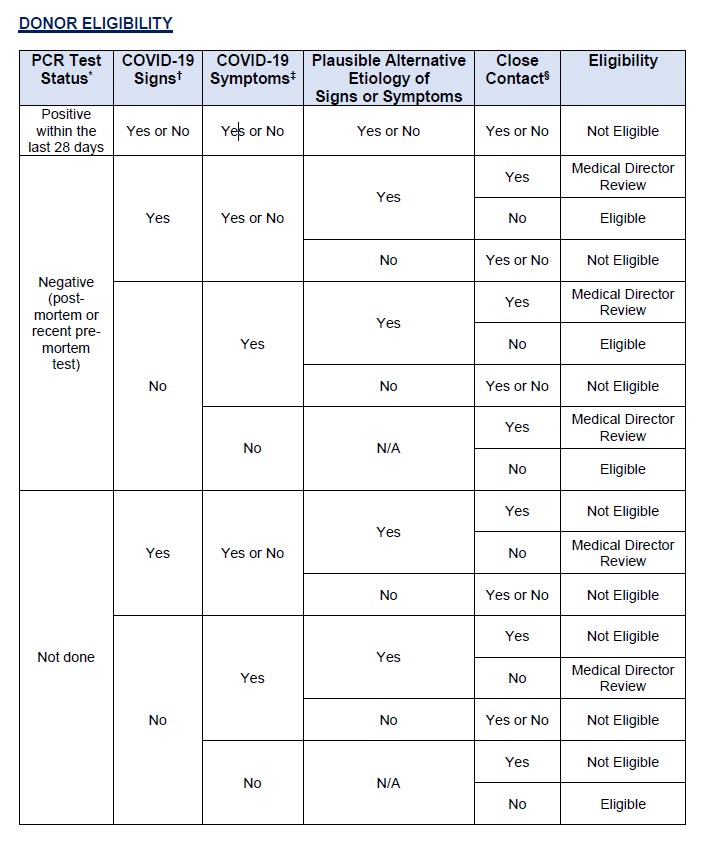
- Donor eligibility criteria remain fluid and complex during the COVID-19 pandemic. Current guidance is more clearly presented in table format for use by Eye Banks and Medical
DONOR TESTING
At this time, the EBAA is not requiring eye banks to perform post-mortem nasopharyngeal (NP) RT-PCR testing for SARS-CoV-2. However, a negative PCR result may be necessary (in addition to a Medical Director Review) to release certain tissue (see Donor Eligibility Table). The decision to not require post-mortem NP RT-PCR testing for SARS-CoV-2 is based on several considerations including the variable false negative rates of current RT-PCR testing, estimated to be approximately 10%. Additionally, diagnostic RT-PCR tests for SARS-CoV have not been validated for cadaveric donors and are not intended for donor screening. Currently, the FDA does not recommend the use of laboratory tests to screen asymptomatic tissue donors.2
The EBAA acknowledges that other associations, hospital systems, eye banks, departments of health, or governments may require that all donors be tested for COVID-19. Eye banks must establish a protocol to ensure access to testing notification and results obtained by partner agencies. Results of such testing must be communicated to end-users on Tissue Report Forms or other supporting documents.
Eye banks may consider post-mortem testing of donors using currently available nasopharyngeal (NP) RT-PCR testing for SARS-CoV-2. Again, these tests have not been validated for cadaveric samples. If testing is performed, results must be obtained prior to release for transplantation and reported to end-users on Tissue Report Forms or other support documents. SARS-CoV-2 testing may reduce, but does not eliminate, the potential of transplanting tissue from a donor with COVID-
- Post-mortem testing must be performed within 24 hours of death. Considerations that may help guide the decision to initiate widespread donor testing should include epidemiologic factors such as the prevalence of disease within the recovery area, and the availability of supplies (e.g. swabs, viral transport media, reagents, etc.).
Finally, the EBAA does not suggest serologic testing for COVID-19 antibodies. Viral RNA can still be detected in patients despite development of antibodies against SARS-CoV-2.3,4
DONOR PREP
A published review5 looked at the persistence of coronaviruses on inanimate surfaces as well as their inactivation with biocidal agents. Their review of the literature found that povidone iodine (0.23 – 7.5%) readily inactivated coronavirus (SARS-CoV and MERS-CoV) infectivity by approximately 4 log10 or more in vitro, with exposure times ranging between 15 seconds and 1 minute. Although we must be careful to extrapolate too much from these findings to the novel coronavirus, SARS-CoV-2, these results certainly support the current EBAA standards for ocular surface prep prior to recovery.
Current EBAA Medical Standard E1.100 Recovery requires double exposure of povidone-iodine to the entire surface of the ocular tissue. This would result in rapid viricidal activity against coronaviruses and reduce the likelihood that COVID-19 may be transmitted through corneal transplantation.
The European Centre for Disease Prevention and Control (ECDC) considers this a disinfection or microbial inactivation step that is validated for enveloped viruses. However, it is not known if infectious virus particles are present inside ocular surface cells or within deeper layers of the ocular tissue that may or may not be eliminated by povidone-iodine preparations.
REFERENCES
1Bayyoud T, Iftner A, Iftner T, et al. Absence of Severe Acute Respiratory Syndrome-Coronavirus- 2 RNA in Human Corneal Tissues. Cornea. 2020 Jun 29 (published online ahead of print).
2Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infection. 2020 Mar;104(3):246-251.
3Updated Information for Human Cell, Tissue, or Cellular or Tissue-based Product (HCT/P) Establishments Regarding the Coronavirus Disease 2019 Pandemic”. US Food & Drug Administration, US Department of Health & Human Services, 2 July 2020,
https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/updated-information- human-cell-tissue-or-cellular-or-tissue-based-product-hctp-establishments.
4To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 Mar 23. doi: 10.1016/S1473-3099(20)30196-1.
5Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 Mar 28. doi: 10.1093/cid/ciaa344.
July 31, 2020
DONOR ELIGIBILITY
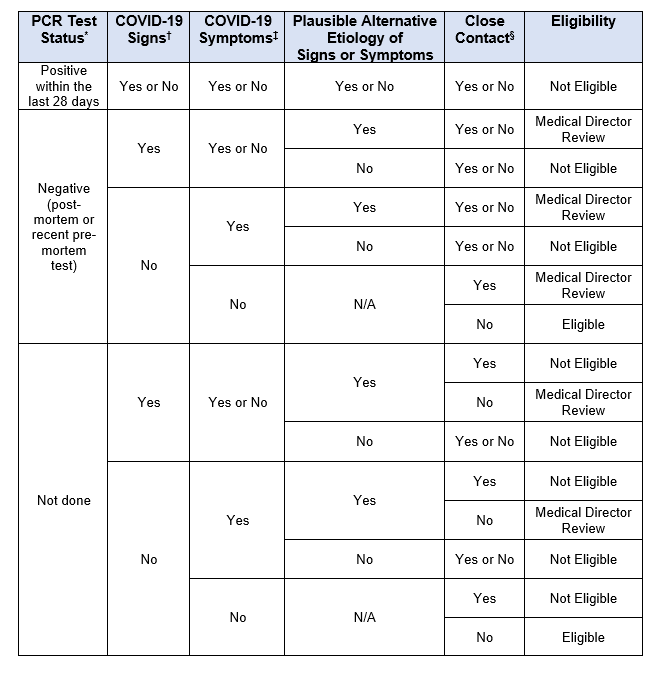
GUIDANCE RATIONALE
The EBAA Policy & Position Review Subcommittee of the Medical Advisory Board continues to update guidance and screening recommendations as the COVID-19 pandemic continues to evolve rapidly. Developments in our understanding of this novel SARS-CoV-2 virus and in our ability to screen donors should allow for the continued provision of safe corneal tissue to patients during this time. As we again proceed with elective corneal transplantation procedures across the US, the safety of corneal tissue may be supported by the following:
- There have been no reported cases of transmission of SARS-CoV-2, MERS-CoV, or any other coronavirus via transplantation of ocular tissue. A recent study reported that no SARS-CoV-2-RNA was detected in the cornea, conjunctiva or aqueous humor of five COVID-19 positive postmortem donors.1
- Current Medical Standards of the EBAA requires use of a double povidone iodine donor prep; povidone iodine has documented in vitro viricidal activity against coronaviruses.
- Increased testing of patients in the hospital and outpatient settings for COVID-19, and greater understanding of COVID-19 symptoms will enhance donor screening and the safety of donor tissue.
- Medical Director review for final determination of donor eligibility in certain cases allows for further assessment of the full clinical picture and/or case specific scenarios.
- Donor eligibility criteria remain fluid and complex during the COVID-19 pandemic. Current guidance is more clearly presented in table format for use by Eye Banks and Medical Directors.
DONOR TESTING
At this time, the EBAA is not requiring eye banks to perform post-mortem nasopharyngeal (NP) RT-PCR testing for SARS-CoV-2. However, a negative PCR result may be necessary (in addition to a Medical Director Review) to release certain tissue (see Donor Eligibility Table). The decision to not require post-mortem NP RT-PCR testing for SARS-CoV-2 is based on several considerations including the variable false negative rates of current RT-PCR testing, ranging between 2-22%. Additionally, diagnostic RT-PCR tests for SARS-CoV have not been validated for cadaveric donors and are not intended for donor screening. Currently, the FDA does not recommend the use of laboratory tests to screen asymptomatic tissue donors.2
The EBAA acknowledges that other associations, hospital systems, eye banks, departments of health, or governments may require that all donors be tested for COVID-19. Eye banks must establish a protocol to ensure access to testing notification and results obtained by partner agencies. Results of such testing must be communicated to end-users on Tissue Report Forms or other supporting documents.
Eye banks may consider post-mortem testing of donors using currently available nasopharyngeal (NP) RT-PCR testing for SARS-CoV-2. Again, these tests have not been validated for cadaveric samples. If testing is performed, results must be obtained prior to release for transplantation and reported to end-users on Tissue Report Forms or other support documents. Tissue from donors with indeterminant, invalid, or inconclusive results should not be released for transplant. SARS-CoV-2 testing may reduce, but does not eliminate, the potential of transplanting tissue from a donor with COVID-19. Post-mortem testing must be performed within 24 hours of death. Considerations that may help guide the decision to initiate widespread donor testing should include epidemiologic factors such as the prevalence of disease within the recovery area, and the availability of supplies (e.g. swabs, viral transport media, reagents, etc.).
Finally, the EBAA does not suggest serologic testing for COVID-19 antibodies. Viral RNA can still be detected in patients despite development of antibodies against SARS-CoV-2.3,4
DONOR PREP
A recently published review2 looked at the persistence of coronaviruses on inanimate surfaces as well as their inactivation with biocidal agents. Their review of the literature found that povidone iodine (0.23 – 7.5%) readily inactivated coronavirus (SARS-CoV and MERS-CoV) infectivity by approximately 4 log10 or more in vitro, with exposure times ranging between 15 seconds and 1 minute. Although we must be careful to extrapolate too much from these findings to the novel coronavirus, SARS-CoV-2, these results certainly support the current EBAA standards for ocular surface prep prior to recovery.
The European Centre for Disease Prevention and Control (ECDC) considers this a disinfection or microbial inactivation step that is validated for enveloped viruses. However, it is not known if infectious virus particles are present inside ocular surface cells or within deeper layers of the ocular tissue that may or may not be eliminated by povidone-iodine preparations.
REFERENCES
1Bayyoud T, Iftner A, Iftner T, et al. Absence of Severe Acute Respiratory Syndrome-Coronavirus-2 RNA in Human Corneal Tissues. Cornea. 2020 Jun 29 (published online ahead of print).
2Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infection. 2020 Mar;104(3):246-251.
3Updated Information for Human Cell, Tissue, or Cellular or Tissue-based Product (HCT/P) Establishments Regarding the Coronavirus Disease 2019 Pandemic”. US Food & Drug Administration, US Department of Health & Human Services, 2 July 2020, https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/updated-information-human-cell-tissue-or-cellular-or-tissue-based-product-hctp-establishments.
4To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 Mar 23. doi: 10.1016/S1473-3099(20)30196-1.
5Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 Mar 28. doi: 10.1093/cid/ciaa344.
July 7, 2020
FDA continues to closely monitor the COVID-19 pandemic and has released updated information for HCT/P establishments. Respiratory viruses, in general, are not known to be transmitted by implantation, transplantation, infusion, or transfer of human cells, tissues, or cellular or tissue-based products (HCT/Ps). To date, there have been no reported cases of transmission of COVID-19 via these products.
Routine screening measures are already in place for evaluating clinical evidence of infection in HCT/P donors. FDA is aware that some HCT/P establishments in the U.S. are considering additional donor screening and testing measures in response to the COVID-19 pandemic.
FDA does not recommend using laboratory tests to screen asymptomatic HCT/P donors.
The HCT/P establishment’s responsible person must determine and document the eligibility of a cell or tissue donor (21 CFR 1271.50). Based on information available at this time, establishments may wish to consider, whether, in the 28 days prior to HCT/P recovery, the donor:
- cared for, lived with, or otherwise had close contact with individuals diagnosed with or suspected of having COVID-19 infection; or
- had been diagnosed with or suspected of having COVID-19 infection; or
- had a positive diagnostic test (e.g., nasopharyngeal swab) for SARS-CoV-2 but never developed symptoms.
New Study Shows Absence of SARS-CoV-2 RNA in Human Corneal Tissues
A pre-publication study in Cornea showed the absence the SARS-CoV-2 viral RNA in corneal tissues obtained from COVID-19 postmortem donors using quantitative (q)RT-PCR-testing. The study was undertaken to examine corneal tissue for SARS-CoV-2 positivity with regard to implications for tissue procurement, processing, corneal transplantation.
German researchers performed (q)RT-PCR-testing on corneal stroma and endothelium, bulbar conjunctiva, conjunctival fluid swabs, anterior chamber fluid and corneal epithelium from 5 patients who expired from COVID-19 with ARDS and multiorgan dysfunction.
In this study no SARS-CoV-2-RNA was detected in conjunctiva, anterior chamber fluid and corneal tissues (endothelium, stroma and epithelium) of COVID-19 donors. This implicates that the risk for SARS-CoV-2 infection via corneal or conjunctival tissue is very low. However, further studies on a higher number of COVID-19 patients are necessary to confirm these results.
May 14, 2020
The EBAA Policy & Position Review Subcommittee of the Medical Advisory Board continues to update guidance and screening recommendations as the COVID-19 pandemic continues to evolve rapidly. Developments in our understanding of this novel SARS-CoV-2 virus and in our ability to screen donors should allow for the continued provision of safe corneal tissue to patients during this time. As we again proceed with elective corneal transplantation procedures across the US, the safety of corneal tissue may be supported by the following:
- There have been no reported cases of transmission of SARS-CoV, MERS-CoV, or any other coronavirus via transplantation of ocular tissue.
- Current Medical Standards of the EBAA requires use of a double povidone iodine donor prep; povidone iodine has documented in vitro viricidal activity against coronaviruses.
- Increased testing of patients in the hospital and outpatient settings for SARS-CoV-2, and greater understanding of COVID-19 symptoms will enhance donor screening and the safety of donor tissue.
- Medical Director review for final determination of donor eligibility in certain cases allows for further assessment of the full clinical picture and/or case specific scenarios.
- Donor eligibility criteria remain fluid and complex during the COVID-19 pandemic. Current guidance is more clearly presented in table format for use by eye banks and Medical Directors.

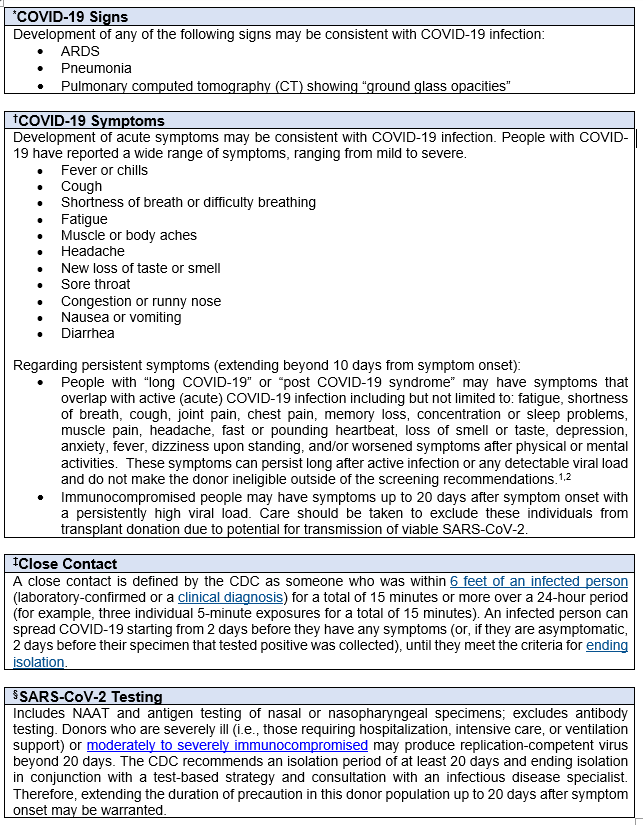
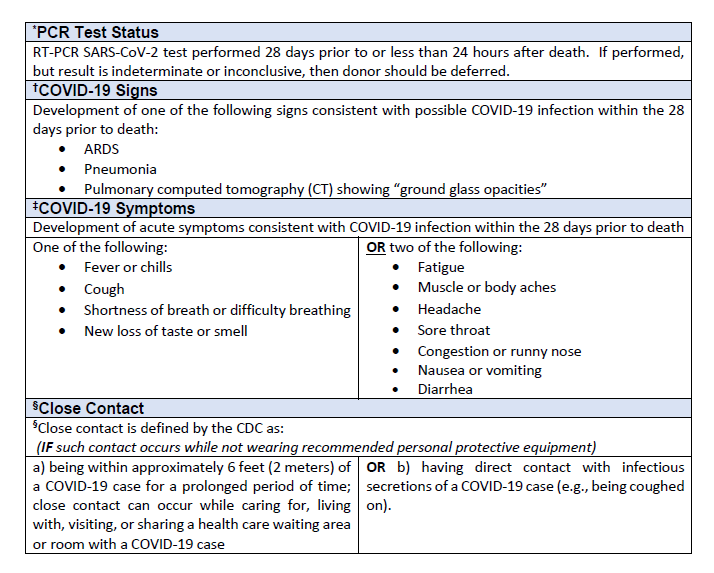
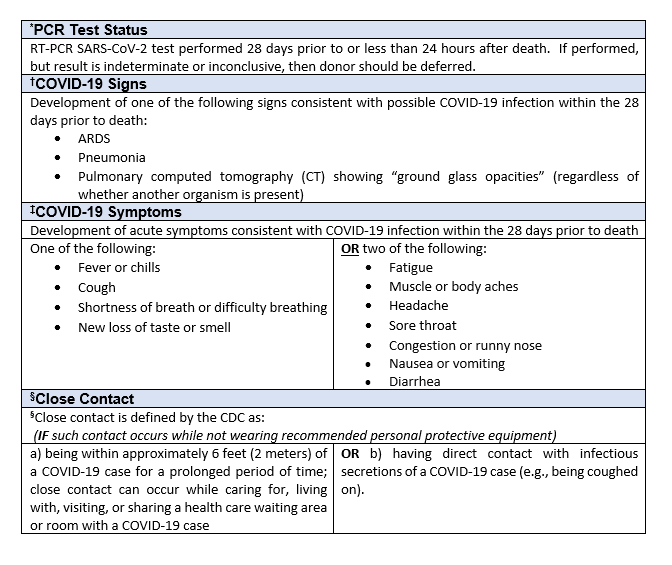
1RT-PCR SARS-CoV-2 test performed prior to or less than 24 hours after death. If performed, but result is indeterminate or inconclusive, then donor should be deferred.
2Development of one of the following signs consistent with possible COVID-19 infection within the 28 days prior to death:
- ARDS
- Pneumonia
- Pulmonary computed tomography (CT) showing “ground glass opacities” (regardless of whether another organism is present)
3Development of acute symptoms consistent with COVID-19 infection within the 28 days prior to death:
One of the following:
- Cough
- Shortness of breath/difficulty breathing
OR
Two of the following:
- Fever
- Chills
- Repeated shaking with chills
- Muscle Pain
- Headache
- Sore throat
- New loss of taste or smell
4Close contact is defined by the CDC as:
- being within approximately 6 feet (2 meters) of a COVID-19 case for a prolonged period of time; close contact can occur while caring for, living with, visiting, or sharing a health care waiting area or room with a COVID-19 case; OR
- having direct contact with infectious secretions of a COVID-19 case (e.g., being coughed on).
IF such contact occurs while not wearing recommended personal protective equipment (PPE).
DONOR TESTING At this time, the EBAA is not requiring eye banks to perform post-mortem nasopharyngeal (NP) RT-PCR testing for SARS-CoV-2. However, a negative PCR result may be necessary (in addition to a Medical Director Review) to release certain tissue (see Donor Eligibility Table). The decision to not require post-mortem NP RT-PCR testing for SARS-CoV-2 is based on several considerations including the variable false negative rates of current RT-PCR testing, ranging between 2-22%. Additionally, diagnostic RT-PCR tests for SARS-CoV have not been validated for cadaveric donors and are not intended for donor screening. Currently, the FDA does not recommend the use of laboratory tests to screen asymptomatic blood or plasma donors.5
The EBAA acknowledges that other associations, hospital systems, eye banks, departments of health, or governments may require that all donors be tested for COVID-19. Eye banks need to establish a protocol to ensure access to testing notification and results obtained by partner agencies. Results of such testing must be communicated to end-users on Tissue Report Forms or other supporting documents.
Eye banks may consider post-mortem testing of donors using currently available nasopharyngeal (NP) RT-PCR testing for SARS-CoV-2. Again, these tests have not been validated for cadaveric samples. If testing is performed, results must be obtained prior to release for transplantation and reported to end-users on Tissue Report Forms or other support documents. Tissue from donors with indeterminant, invalid, or inconclusive results should not be released for transplant. SARS-CoV-2 testing may reduce, but does not eliminate, the potential of transplanting tissue from a donor with COVID-19. Post-mortem testing must be performed within 24 hours of death. Considerations that may help guide the decision to initiate wide-spread donor testing should include epidemiologic factors such as the prevalence of disease within the recovery area, and the availability of supplies (e.g. swabs, viral transport media, reagents, etc.).
Finally, the EBAA does not suggest serologic testing for COVID-19 antibodies. Viral RNA can still be detected in patients despite development of antibodies against SARS-CoV-2.6,7
DONOR PREP A recently published review8 looked at the persistence of coronaviruses on inanimate surfaces as well as their inactivation with biocidal agents. Their review of the literature found that povidone iodine (0.23 – 7.5%) readily inactivated coronavirus (SARS-CoV and MERS-CoV) infectivity by approximately 4 log10 or more in vitro, with exposure times ranging between 15 seconds and 1 minute. Although we must be careful to extrapolate too much from these findings to the novel coronavirus, SARS-CoV-2, these results certainly support the current EBAA standards for ocular surface prep prior to recovery.
The European Centre for Disease Prevention and Control (ECDC) considers this a disinfection or microbial inactivation step that is validated for enveloped viruses. However, it is not known if infectious virus particles are present inside ocular surface cells or within deeper layers of the ocular tissue that may or may not be eliminated by povidone-iodine preparations.
5Kampf G, Todt D, Pfaender S, et al. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J Hosp Infection. 2020 Mar;104(3):246-251.
6Updated Information for Human Cell, Tissue, or Cellular or Tissue-based Product (HCT/P) Establishments Regarding the Coronavirus Disease 2019 Pandemic”. US Food & Drug Administration, US Department of Health & Human Services, 1 April 2020, https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/updated-information-human-cell-tissue-or-cellular-or-tissue-based-product-hctp-establishments.
7To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020 Mar 23. doi: 10.1016/S1473-3099(20)30196-1.
8Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 Mar 28. doi: 10.1093/cid/ciaa344.
April 29, 2020
The global case total climbed to 3,094,829 in 185 nations by Tuesday night, with fatalities reaching 215,461, according to the Johns Hopkins online dashboard. The US total is at 1,004,908 with 57,812 deaths.
Preliminary results from serologic surveys suggest that coronavirus infections greatly outnumber confirmed COVID-19 cases by a factor of 10 or more. Higher infection rates mean that there is a much lower infection fatality rate than the 6 percent case fatality rate seen globally and, in the U.S.,
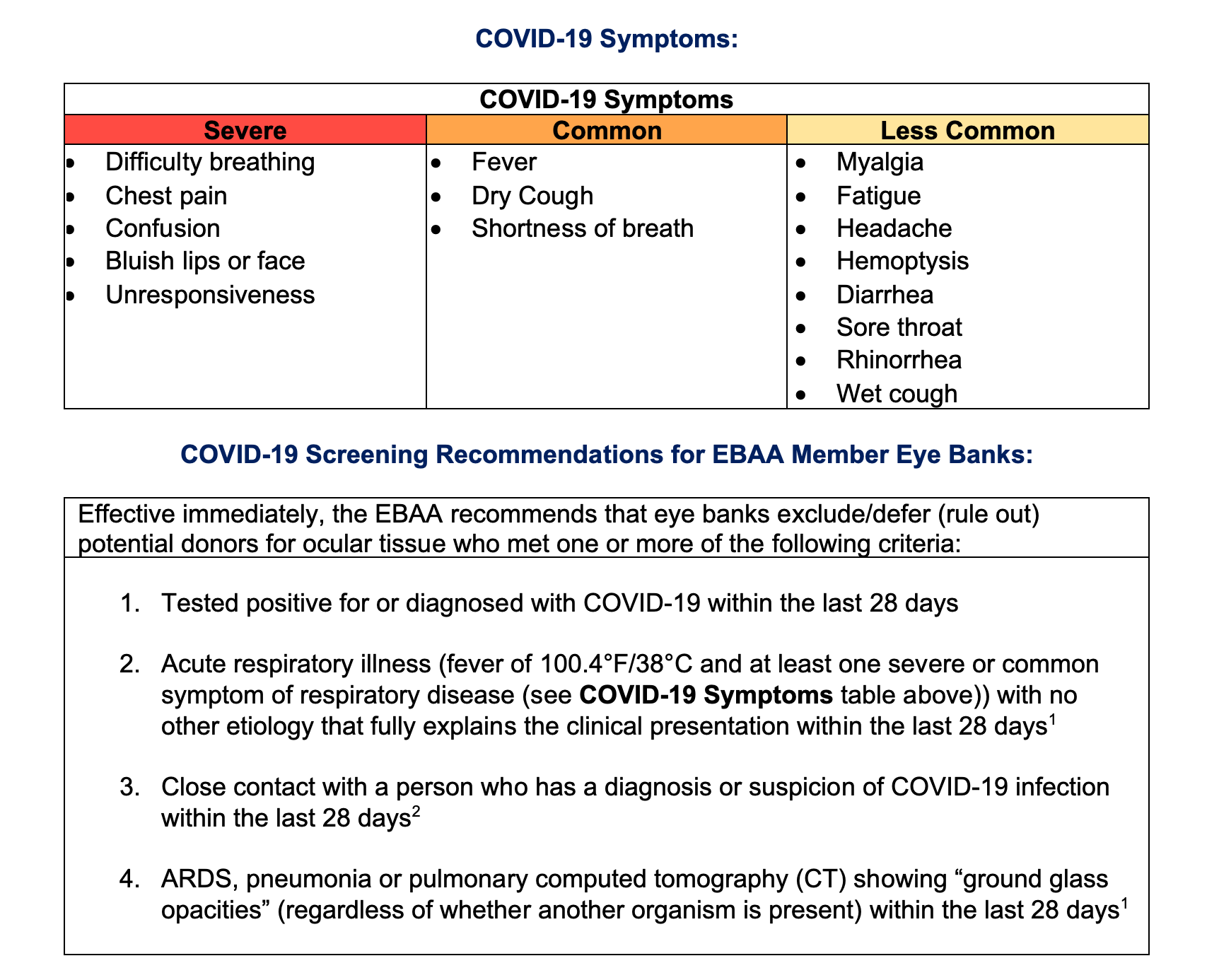
COVID-19 Symptoms
The CDC added six new potential COVID-19 symptoms which typically appear 2-14 days after exposure. Originally listing only fever, cough and shortness of breath as coronavirus symptoms, the agency has now added chills, repeated shaking with chills, muscle pain, headache, sore throat and new loss of taste or smell.
Prolonged SARS-CoV-2 RNA Detection from Ocular Sections
A single case report published in the Annals of Internal Medicine,has demonstrated presence of infectious SARS-CoV-2 virus and viral RNA in the conjunctiva of a patient with a history of conjunctivitis up to 27 days. The first patient in Italy to be diagnosed with COVID-19 also had conjunctivitis in addition to fever and respiratory and gastrointestinal signs. RT-PCR on conjunctival swabs showed SARS-CoV-2 RNA from day 3 of hospitalization until day 21 (1 day after the conjunctivitis resolved), and again at day 27, at which point nasal swabs were negative. Infectious virus was isolated by cell culture from a sample taken on day 3.
Povidone-Iodine and COVID-19
A recent review about the persistence of coronaviruses on inanimate surfaces, as well as their inactivation with biocidal agents revealed that povidone iodine (0.23 – 7.5%) readily inactivated coronavirus infectivity by approximately 4 log10or more, with exposure times of 15 seconds.
Current EBAA Medical Standard E1.100 Recovery procedures requiring double exposure of povidone-iodine to ocular tissue would result in rapid viricidal activity against coronaviruses and reduce the likelihood that COVID-19 may be transmitted through corneal transplantation.
The ocular surface is not an inanimate surface and it is not known if infectious virus particles inside ocular surface cells are eliminated by povidone iodine preparations. Nonetheless, the European Centre for Disease Prevention and Control (ECDC) considers this a disinfection or microbial inactivation step that is validated for enveloped viruses.
Latest Guidance and Publications on COVID-19:
The CDC has developed a new web page to provide summaries of hospitalization data due to COVID-19 in the U.S. See the COVID-NET page.
Information for Healthcare Professionals
Clinical Care Guidance for Healthcare Professionals about COVID-19
Guidance for U.S. Healthcare Facilities about COVID-19
COVID-19 Infection Prevention and Control in Healthcare Settings: Questions and Answers
Characteristics of Health Care Personnel with COVID-19 — United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:477–481. DOI: http://dx.doi.org/10.15585/mmwr.mm6915e6
Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA. Published online April 15, 2020. doi:10.1001/jama.2020.6266 https://jamanetwork.com/journals/jama/fullarticle/2764787
Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. Published online April 22, 2020. doi:10.1001/jama.2020.6775 https://jamanetwork.com/journals/jama/fullarticle/2765184
Chow EJ, Schwartz NG, Tobolowsky FA, et al. Symptom Screening at Illness Onset of Health Care Personnel With SARS-CoV-2 Infection in King County, Washington. JAMA. Published online April 17, 2020. doi:10.1001/jama.2020.6637 https://jamanetwork.com/journals/jama/fullarticle/2764953
Chen L, Liu M, Zhang Z, et al.Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. British Journal of Ophthalmology Published Online First: 07 April 2020. doi: 10.1136/bjophthalmol-2020-316304 https://bjo.bmj.com/content/early/2020/04/07/bjophthalmol-2020-316304
April 14, 2020
COVID-19 Updated Guidance and Screening Recommendations
1 Tissue from a patient who has tested negative for COVID-19 by any of the available testing methods AND has another etiology which explains the symptoms and/or findings, may be considered for transplant use with Medical Director approval.
2 Close contact is defined as: a) being within approximately 6 feet (2 meters) of a COVID-19 case for a prolonged period of time; close contact can occur while caring for, living with, visiting, or sharing a health care waiting area or room with a COVID-19 case; or b) having direct contact with infectious secretions of a COVID-19 case (e.g., being coughed on).
The number of COVID-19 cases in the United States passed 584,074 and deaths topped 24,485 making it the world’s deadliest outbreak of COVID-19. At the global level, the total reached 1,949,210 cases today from 210 countries with 123,348 deaths reported, according to the Johns Hopkins online dashboard.
EBAA is pleased to provide the free recording of the April 3rd webinar, “EBAA COVID-19 Update” on eyeLEARN to our members. The 90-minute informational session features discussion and Q&A on COVID-19, and what eye banks need to know about this novel disease. If you missed the session, we encourage you to watch the recording today.
April 2, 2020
The Food and Drug Administration (FDA) shared updated information for human cells, tissues, or cellular or tissue-based products (HCT/P) establishments regarding the Cornonavirus-19 2019 Pandemic.
While respiratory viruses, in general, are not known to be transmitted by implantation, transplantation, infusion, or transfer of human cells, tissues, or cellular or tissue-based products (HCT/Ps), the potential for transmission of COVID-19 by HCT/Ps is unknown at this time. There have been no reported cases of transmission of COVID-19 via these products.
FDA is aware that some HCT/P establishments in the U.S. are considering additional donor screening and testing measures in response to the COVID-19 outbreak.
At this time, FDA does not recommend establishments use laboratory tests to screen asymptomatic HCT/P donors. Based on available information, it appears that SARS-CoV2 has only been detected in blood samples of a small percentage of severely ill patients.
The HCT/P establishment’s responsible person must evaluate a prospective donor and determine eligibility (21 CFR 1271.50). Based on the limited information available at this time, establishments may wish to consider, whether, in the 28 days prior to HCT/P recovery, the donor
- cared for, lived with, or otherwise had close contact with individuals diagnosed with or suspected of having COVID-19 infection; or
- been diagnosed with or suspected of having COVID-19 infection.
For HCT/Ps regulated as biological products under Section 351 of the Public Health Service Act, FDA is continually assessing available scientific evidence, and evaluating benefits and risks, to determine whether SARS-CoV-2 testing is warranted on certain types of HCT/Ps used in the manufacture of a biological product and/or warranted for the final product.
March 30, 2020
President Trump extends social distancing guidance until April 30
President Trump extends social distancing guidance until April 30after the nation’s top epidemiologist, Dr. Anthony Fauci says COVID-19 could claim between 100,000 and 200,000 American lives.
The CDC, citing “extensive community transmission,” insisted that residents in New Jersey, Connecticut and New York refrain from non-essential travel for 14 days, effective immediately. CDC has also released updated COVID-19 travel recommendations by country.
Eye banks are recognized by the Federal Emergency Management Agency under Emergency Support Function #8 as critical public health and medical services. EBAA has prepared a letter for recovery technicians to carry to travel on closed roads or to enter hospitals, morgues or other buildings.
FDA has recently approved a rapid SARS-CoV-2 point-of-care diagnostic test, but priorities for testing do not include those with mild or no respiratory symptoms. CDC has updated their guidance for Collection and Submission of Postmortem Specimens from Deceased Persons with Known or Suspected COVID-19,
However, as the demand for tissue is low at present, if there is suspicion that a potential donor may have had COVID-19 based on history or other factors, even in the setting of a negative test, the PPRS would recommend deferring recovery. Therefore, our guidance remains unchanged.

March 19, 2020
CMS releases Elective Surgery Recommendations during the COVID-19 pandemic, recommending that elective surgeries and non-essential medical and surgical procedures should be delayed to conserve critical resources and limit exposure of patients and staff to SARS-CoV-2 virus.
CDC published the first description of outcomes among patients with COVID-19 in the United States.
March 17, 2020
EBAA continues to closely monitor the outbreak of respiratory disease
caused by a novel coronavirus first identified in Wuhan, Hubei Province, China and which has now been detected in 152 countries and territories. The virus has been named “SARS-CoV-2” and the disease it causes has been named “Coronavirus Disease 2019” (abbreviated “COVID-19”).
The Policy and Position Review Subcommittee (PPRS) of the Medical Advisory Board continues to meet to assess the clinical risks of the novel coronavirus (COVID-19), including the potential for person to person transmission, and risks for disseminated infection from ocular tissue. This updated guidance provides insight into the current issues potentially impacting tissue safety.
Key Points about COVID-19
- The World Health Organization (WHO) now considers the outbreak of novel coronavirus disease 2019 (COVID-19) to be a pandemic and the CDC issued a Global COVID-19 Outbreak Notice1that classified the current situation as sustained community-level transmission.
- While respiratory viruses, in general, are not known to be transmitted by transplantation of human cells, tissues, or cellular or tissue-based products (HCT/Ps), the potential for transmission of COVID-19 by HCT/Ps is unknown at this time. There have been no reported cases of transmission of COVID-19 via these products2.
- A study has shown COVID-19 may be detected in tears and conjunctival secretions of patients with COVID-19 pneumonia and conjunctivitis. This suggests transplanted ocular tissue may be at risk of transmitting COVID-19 to recipients3,4.
- The virus appears to spread primarily via respiratory droplets produced when an infected person coughs or sneezes.It also could be spread if people touch an object or surface on which virus is present from an infected person, and then touch their mouths, noses or eyes4.
- A study of more than 72,000 COVID-19 patients reveals a case-fatality rate of 2.3% and suggests most cases are mild, but the disease is most severe in the elderly5.
- Though the death rate is higher in the elderly, there is also a correlation to higher death rates among individuals with certain vulnerable co-morbidities, including: diabetes, heart disease, receiving immunosuppressive medication, chronic lung disease, and chronic kidney disease6.
- The S. Surgeon General Jerome Adams and the American College of Surgeons are advising hospitals and ASCs to consider postponing or canceling elective surgeries, which may lead to a short-term decrease in ocular tissue demand7.8.

Key Points About Existing Screening Processes (US Eye Banks)
- The existing Uniform Donor Risk Assessment Interview (DRAI) contains screening capture questions for generalized infection symptoms (Q6) that can be used effectively to screen a potential donor with COVID-19symptoms.
- The existing Uniform DRAI contains screening capture questions for generalized travel history (Q26, Q27, and Addendum “6 month” travel question (QZ3)) that can be used effectively to screen for a history of travel to a COVID-19 endemic region.
- The existing Uniform DRAI does not contain specific questions asking about COVID-19 infection or exposure, which may warrant adding questions to screen for these details.
- Some eye bank data systems have added addendum questions to screen for specific COVID-19 exposure or travel risk. Eye banks are reminded that the DRAI process is a dialogue and that questions asked should be adjusted according to the responses received.
- Eye banks should document the risk assessment for COVID-19 infection and ensure all staff are aware of the above exclusionary criteria.
The deferral period of 28 days represents twice the maximum reported incubation period (14 days) from exposure to onset of symptoms, which is the surveillance interval being used in public health reporting by CDC.
We are not at this time advocating a change to the Medical Standards or a required addendum to the Uniform DRAI. It is incumbent on the eye bank to execute screening criteria to protect the public from COVID-19. Following this guidance is voluntary. Should an eye bank implement alternative criteria to achieve the same goal, the eye bank must keep that documentation on file along with the rationale for later inspection.
The PPRS will continue to monitor the rapidly changing information for evidence suggesting risk for COVID-19 as a transplantation-transmitted infection and the EBAA will provideupdates as warranted. Members with questions may contact Jennifer@restoresight.org.
References and Resources:
- Centers for Disease Conrol and Prevention.Global COVID-19 Outbreak Notice. https://wwwnc.cdc.gov/travel/notices/alert/coronavirus-global
- S. Food and Drug Administration. Coronavirus Disease 2019 (COVID-19) Frequently Asked Questions. https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/coronavirus-disease-2019-covid-19-frequently-asked-questions
- Xia J, Tong J, Liu M, Shen Y, Guo D. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS?CoV?2 infection. J Med Virol. 2020;1–6. https://doi.org/10.1002/jmv.25725
- American Academy of Ophthalmology. Member Alert: Important Coronavirus Updates for Ophthalmologists, March 16, 2020.https://www.aao.org/headline/alert-important-coronavirus-context
- Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72?314 Cases From the Chinese Center for Disease Control and Prevention. https://jamanetwork.com/journals/jama/fullarticle/2762130
- Zhou, F, Yu, T, Du R, Fan G, Liu, Y, Liu, Z, Xiang, J, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet, Published online March 9, 2020 https://doi.org/10.1016/S0140-6736(20)30566-3
- @Surgeon_General. “Hospital & healthcare systems, PLEASE CONSIDER STOPPING ELECTIVE PROCEDURES until we can #FlattenTheCurve!“ Twitter, 14 Mar. 2020, 8:07 a.m. https://twitter.com/surgeon_general?lang=en
- American College of Surgeons. COVID-19: Recommendations for Management of Elective Surgical Procedures. https://www.facs.org/about-acs/covid-19/information-for-surgeons
- Centers for Disease Conrol and Prevention. Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19). https://www.cdc.gov/coronavirus/2019-nCoV/hcp/clinical-criteria.html
- World Health Organization Coronavirus disease (COVID-2019) Situation Report 55 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200316-sitrep-56-covid-19.pdf?sfvrsn=9fda7db2_2
March 9, 2020
CDC Releases Updated Guidance on Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19)
CDC issued the following Health Alert Network (HAN) Health Update on March 08, 2020: regarding evaluating and identifying patients who should be tested for COVID-19:
https://emergency.cdc.gov/han/2020/han00429.asp?deliveryName=USCDC_511-DM22015
With increased access to testing, CDC has expanded the criteria for testing for COVID-19 to include more symptomatic persons, even in the absence of travel history to affected areas or known exposure to another case.
This will allow public health to quickly detect and respond to community spread of the virus in the United States.
International Areas with Sustained (Ongoing) Transmission include:
- China: Level 3 Travel Health Notice
- Iran: Level 3 Travel Health Notice
- Italy: Level 3 Travel Health Notice
- Japan: Level 2 Travel Health Notice
- South Korea: Level 3 Travel Health Notice
As of March 9, 2020, the U.S. has confirmed at least 566 cases of COVID-19 across 34 states and has confirmed 22 deaths from the illness.
March 2, 2020
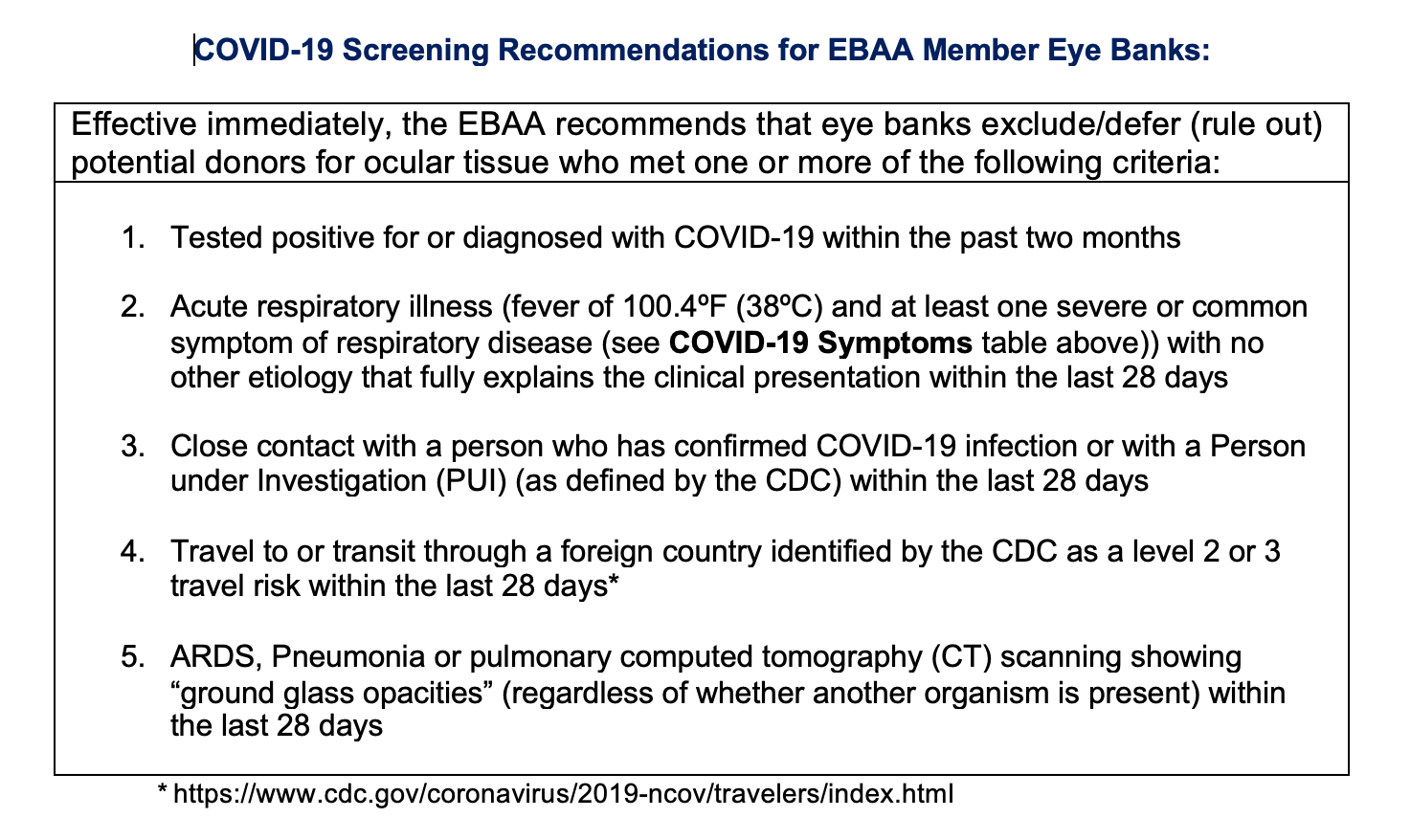
EBAA has released updated COVID-19 Screening Recommendations for EBAA Member Eye Banks.
February 28, 2020
The Centers for Disease Control and Prevention (CDC) revised their Criteria to Guide Evaluation of PUI for COVID-19 on February 27, 2020.
This can be found at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html
| Clinical Features | & | Epidemiologic Risk |
| Fever1 or signs/symptoms of lower respiratory illness (e.g. cough or shortness of breath) | AND | Any person, including health care workers2, who has had close contact3 with a laboratory-confirmed4 COVID-19 patient within 14 days of symptom onset |
| Fever1 and signs/symptoms of a lower respiratory illness (e.g., cough or shortness of breath) requiring hospitalization | AND | A history of travel from affected geographic areas5 (see below) within 14 days of symptom onset |
| Fever1 with severe acute lower respiratory illness (e.g., pneumonia, ARDS) requiring hospitalization4 and without alternative explanatory diagnosis (e.g., influenza)6 | AND | No source of exposure has been identified |
Affected Geographic Areas with Widespread or Sustained Community Transmission will be defined as a country with at least a CDC Level 2 Travel Health Notice. See all COVID-19 Travel Health Notices.
As of February 26, 2020, this includes:
- China
- Iran
- Italy
- Japan
- South Korea
EBAA continues to closely monitor the outbreak of respiratory illness caused by coronavirus 2019 (COVID-19).
The Policy and Position Review Subcommittee (PPRS) of the Medical Advisory Board is currently working to revise our COVID-19 Screening Recommendations for Eye Banks.
February 27, 2020
A person in Northern California has contracted the coronavirus with no known exposure to the virus through travel or close contact with a known infected individual.
There are now more new cases reported from countries outside of China than from China.
CDC has issued the following travel guidance related to COVID-19:
- China — Level 3, Avoid Nonessential Travel — updated February 22;
- South Korea — Level 3, Avoid Nonessential Travel — updated February 24;
- Japan — Level 2, Practice Enhanced Precautions — updated February 22;
- Iran — Level 2, Practice Enhanced Precautions — issued February 23;
- Italy — Level 2, Practice Enhanced Precautions — issued February 23;
- Hong Kong — Level 1, Practice Usual Precautions — issued February 19.
CDC also recommends that all travelers reconsider cruise ship voyages into or within Asia at this time.
February 25, 2020:
Updated Travel Advisories from CDC:
Level 3 Travel Health Notice for Coronavirus in South Korea.
Level 2 Travel Health Notices for Coronavirus in Italy and Iran.
February 24, 2020:
CDC has issued a Level 1 Travel Notice for Hong Kong, Iran and Italy.
Other countries with apparent community spread include Singapore, Taiwan, Thailand, and Vietnam.
There have been 14 confirmed cases of COVID-19 in the United States, including 12 travel related cases and 2 cases of person-to-person spread. There are 39 cases among persons repatriated to the U.S. via State Department-chartered flights: 3 from Wuhan, China and 36 from the Diamond Princess Cruise Ship.
February 21, 2020
CDC has issued a level 1 travel notice for Japan because of coronavirus.
February 19, 2020
CDC issued a travel notice for Hong Kong associated with COVID-19. Defer any donor who has traveled to mainland China or Hong Kong in the past 28 days.
February 18, 2020
The Food and Drug Administration (FDA)
shared important information for human cells, tissues, or cellular or tissue-based products (HCT/P) establishments regarding the novel coronavirus outbreak this week. While respiratory viruses, in general, are not known to be transmitted by implantation, transplantation, infusion, or transfer of HCT/Ps, the potential for transmission of COVID-19 by HCT/Ps is unknown at this time. There have been no reported cases of transmission of COVID-19 via these products.
Routine screening measures are already in place for evaluating clinical evidence of infection in HCT/P donors. Establishments may wish to consider the following donor history in the 28 days prior to HCT/P recovery for persons who have:
- traveled to areas with COVID-19 outbreaks, as defined by CDC
- lived with individuals diagnosed with or suspected of having COVID-19 infection; or
- been diagnosed with or suspected of having COVID-19 infection.
The official name for the disease caused by the 2019 novel coronavirus is “COVID-19,” officials from the World Health Organization (WHO) announced in a media briefing.
February 17, 2020:
- Globally there have been 71,902 confirmed cases (1,775 deaths) of COVID-19. The vast majority of the cases are inside China; about 794 cases have been confirmed in at least 25 other countries, with 3 deaths.
- CDC Confirms the 15th Case of Coronavirus Disease (COVID-19) in the United States
- 454 confirmed cases have been aboard the Diamond Princess cruise ship, including 14 Americans who tested positive after being evacuated.
- About 82 percent of the cases — including all cases in the United States — have been mild, with symptoms that require little or no medical intervention
- The CDC released new infection control guidelines for treating coronavirus in healthcare settings.
- China’s National Health Commission said more than 1,700 medical workers have been infected with coronavirus, six of whom have died.
- Early study suggests no COVID-19 vertical transmission – passing infection from mother to infant in utero occurs.
- HRSA released an information sheet for Organ Procurement and Transplantation Network OPTN – Information for transplant programs and OPOs regarding 2019 Novel Coronavirus
For the latest information about 2019 Novel Coronavirus (2019-nCoV), visit these sites:
- CDC 2019-nCoV Information for Healthcare Professionals
- CDC 2019-nCoV home page
- CDC interim guidance on 2019-nCoV
- CDC Health Alert Network
- WHO COVID-19 Situation Reports
- APIC Novel Coronavirus Fact Sheet
- Tracking COVID-19 Global Cases by Johns Hopkins CSSE
February 11, 2020
On February 11, 2020 the World Health Organization announced an official name for the disease that is causing the 2019 novel coronavirus outbreak, COVID-19.
“COVI” comes from coronavirus. The “D” stands for disease. The 19 represents 2019, the year the virus was first identified, in December. The name will apply for the entire spectrum of cases, from mild to severe, according to a WHO spokesperson.
EBAA released the 2019-nCoV Screening Recommendations for EBAA Member Eye Banks on February 3, 2020 and our recommendations remain unchanged.
For the latest information about 2019 Novel Coronavirus (2019-nCoV), visit these sites: